The times of the industry
Mailén Agüero – Business Development Analyst
Francisco Stefano – Director
The arrival of a drug on the market has many challenges.
The development of a drug is a long and complex process, which can last between 10 and 15 years from the discovery of a candidate molecule to its marketing authorization by the regulatory agency.
However, when we are talking about a generic pharmaceutical product, it takes approximately 6 to 7 years to reach the market.
Every drug must go through a rigorous research and quality process. This is a fundamental requirement for its subsequent commercialization and administration in people.
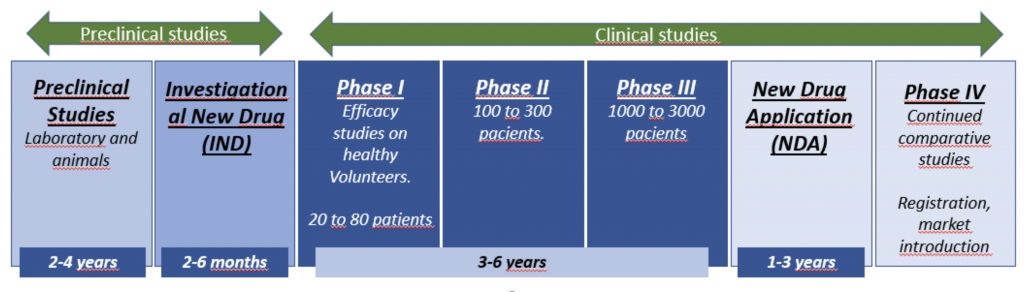
- Preclinical studies
- Investigational New Drug (IND) Application
- Clinical trials: Phase I, II, and III
- New Drug Application (NDA)
- Phase IV studies
Preclinical studies
A pharmaceutical company conducts certain studies before the future drug is administered to a human being. Laboratory and animal studies should be performed to demonstrate the biological activity of the drug against the target disease. The safety of the drug must also be evaluated. These tests take an average of 3 1/2 years.
Investigational New Drug Application (IND)
The pharmaceutical company files an Investigational New Drug (IND) application with the regulatory agency to begin testing the drug in people. The IND becomes effective if the regulatory agency does not disapprove it within 30 days. The IND must include the following information: the results of previous experiments; how, where and by whom the new studies will be carried out; the chemical structure of the compound; how it is believed to work in the body; any toxic effects found in animal studies; and how the compound is made.
Clinical Trials
Phase I: Phase I studies are usually the first tests of a drug under development in healthy volunteers. These studies involve around 20 to 80 volunteers. Testing determines the safety of a drug, including the safe dose range, as well as how the drug is absorbed, distributed, metabolized, and excreted, and the duration of its action. Phase I trials take an average of 1 year.
Phase II: These studies are carried out in patients with the disease for which the drug is intended. This phase is usually designed to identify abnormalities are the minimum and maximum doses. Trials generally involve 100 to 300 patient volunteers and are controlled in design. They are performed to assess the effectiveness of the drug. Phase II usually lasts about 2 years.
Phase III: These are the large definitive randomized trials that are submitted to the regulatory agency to get a drug approved. This phase examines the efficacy and safety (adverse events) of the new drug. Phase III trials typically involve 1,000 to 3,000 patients in clinics and hospitals. Patients are often asked to list possible side effects, often stemming from what was seen in phase II studies. Patients are also free to report any other side effects they experience while taking the new drug or the placebo (the “sugar pill” given to a percentage of patients in a trial study). Phase III takes an average of 3 years.
New Drug Application (NDA)
After phase III clinical trials, all study data is analyzed and an NDA is filed with the regulatory agency (provided the data appears to demonstrate the safety and efficacy of the drug). The NDA contains all the data collected to date on the medicine. (An NDA typically consists of at least 100,000 pages.) The average NDA review time is 30 months (2 1/2 years).
Phase IV studies
Phase IV is any organized collection of data from patients who are taking a drug that has already received approval by the regulatory agency. In phase IV studies, patients can check boxes on a list (as in phase III studies) or they can simply report other symptoms.
Generic Drugs
Because generic drugs are comparable to drugs already on the market, clinical trials are not conducted to prove that your product is safe and effective. Instead, conduct bioequivalence studies and submit an abbreviated new drug application (ANDA)
There is a third option called 505(b)(2) / Hybrid, find more information in our director’s article:
“Transdermal Drug Delivery Systems and 505(b)(2) / Hybrid Regulatory Pathways.”
Subscribe to
#AmarinNews
Partner with us!
We offer expertise and experience, together with flexibility and the ability to adapt to your needs.
- info@amarintech.com.ar
- +54 11 4588-6500
- Sanchez 2045 (C1416BQG), Buenos Aires, Argentina.

